HYBRID KOL Event on Eneboparatide: A PTHR1 Agonist for Hypoparathyroidism
June 16, 2023 | 8:30 am CT (9:30 am ET)
Location: Sheraton Grand Chicago Riverwalk Chicago, IL
Advancing peptides for the treatment of rare endocrine and related diseases
We are a clinical stage, global company, building on our team’s established expertise to transform the lives of patients living with rare endocrine and related diseases.
PROGRAMS
Eneboparatide
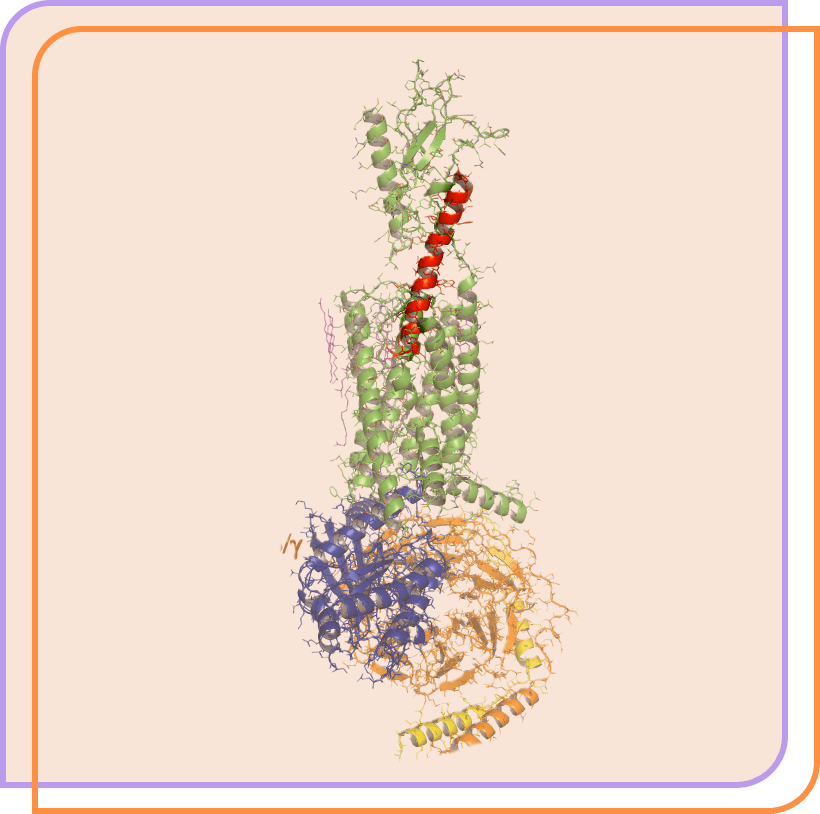
Our lead clinical candidate is a potentially clinically superior therapeutic peptide in development for hypoparathyroidism.
Eneboparatide (red) bound to the PTH1 receptor. Adapted from Zhao et al. Science 364:138, 2019.
Image generated with the PyMOL Molecular Graphics System, Version 2.3.2 Schrödinger, LLC.
AZP-3813
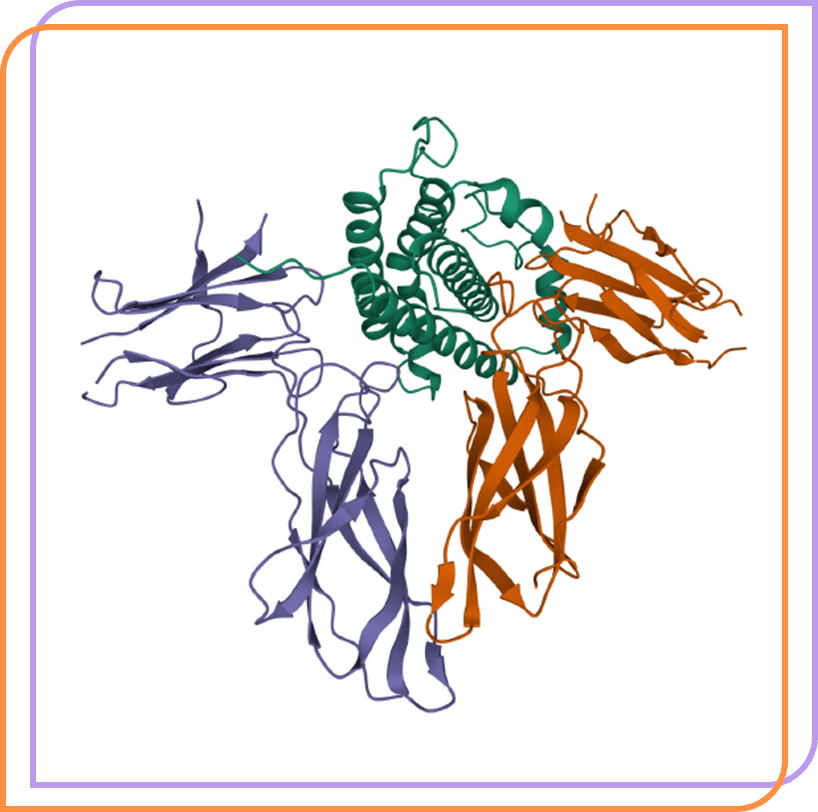
AZP-3813 is a highly potent, peptide growth hormone (GH) receptor antagonist in development for acromegaly.
HUMAN GROWTH HORMONE AND EXTRACELLULAR DOMAIN OF ITS RECEPTOR: CRYSTAL STRUCTURE OF THE COMPLEX. de Vos, A.M., Ultsch, M., Kossiakoff, A.A. (1992) Science 255: 306-312. Image created with Mol* (D. Sehnal, A.S. Rose, J. Kovca, S.K. Burley, S. Velankar (2018) Mol*: Towards a common library and tools for web molecular graphics MolVA/EuroVis Proceedings. doi:10.2312/molva.20181103). RCSB Protein Data Bank, PDB ID: 3HHR.

LEADERSHIP
Our management team has the collective and proven expertise necessary to advance its portfolio of innovative therapies in the clinic.
Press Releases & Recent News
AstraZeneca Closes Acquisition of Amolyt Pharma
July 15, 2024
Download a PDF copy of this press release: EN FR Lyon, France, and Cambridge, MA, July 15, 2024 — Amolyt ...
Read More →
Written by
jpoly4
Amolyt Pharma Announces Abstracts Accepted for Presentation at the GRS Congress, ECE and ENDO
May 7, 2024
Download a PDF copy of this press release: EN FR Lyon, France, and Cambridge, MA, May 7, 2024 — Amolyt ...
Read More →
Written by
Dan Hennings
