Eneboparatide: Hypoparathyroidism
Eneboparatide is a parathyroid hormone receptor 1 (PTHR1) agonist with a novel mechanism of action rationally designed to meet the therapeutic goals of hypoparathyroidism. It activates a specific conformation of the PTH1 receptor to achieve three key therapeutic goals: 1) safely produce sustained and stable levels of calcium in the blood to effectively address symptoms while removing oral calcium and active Vitamin D supplements, 2) normalize urine calcium excretion to prevent kidney disease, and 3) restore bone turnover to a more physiologic state while preventing excessive bone resorption over formation to help preserve bone integrity.
Eneboparatide has demonstrated an optimal pharmacological profile1,2 and has successfully completed pre-investigational new drug activities. A Phase 1 clinical trial in healthy volunteers demonstrated that eneboparatide induced a rapid, dose-dependent increase in serum calcium levels that was sustained and stable over the treatment period. In addition, no increase in urinary calcium excretion was observed, and bone biomarkers were unchanged.
Data from a Phase 2a trial showed that eneboparatide was well-tolerated, and daily administration enabled 93% of patients to discontinue standard of care therapy (calcium and vitamin D supplementation) while mean serum calcium was maintained within the target range. Mean urinary calcium excretion was rapidly normalized, including in all but one patient with elevated urinary calcium at baseline, and bone biomarkers increased in line with a resumption of a normalized physiological level of bone turnover. Consistent with the findings in bone biomarkers, bone mineral density (BMD) was stable, including in patients with osteopenia.
- Shimizu, et al. “Pharmacodynamic Actions of a Long-Acting PTH Analog (LA-PTH) in Thyroparathyroidectomized (TPTX) Rats and Normal Monkeys.” J Bone Miner Res. 2016 Jul;31(7):1405-12.
- Bi, et al. “Diphtheria Toxin- and GFP-Based Mouse Models of Acquired Hypoparathyroidism and Treatment With a Long-Acting Parathyroid Hormone Analog.” J Bone Miner Res. 2016 May;31(5):975-84.
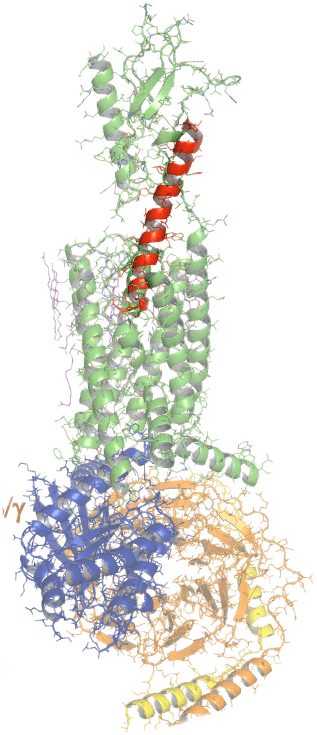
Eneboparatide (red) bound to the PTH1 receptor. Adapted from Zhao et al. Science 364:138, 2019.
Image generated with the PyMOL Molecular Graphics System, Version 2.3.2 Schrödinger, LLC.
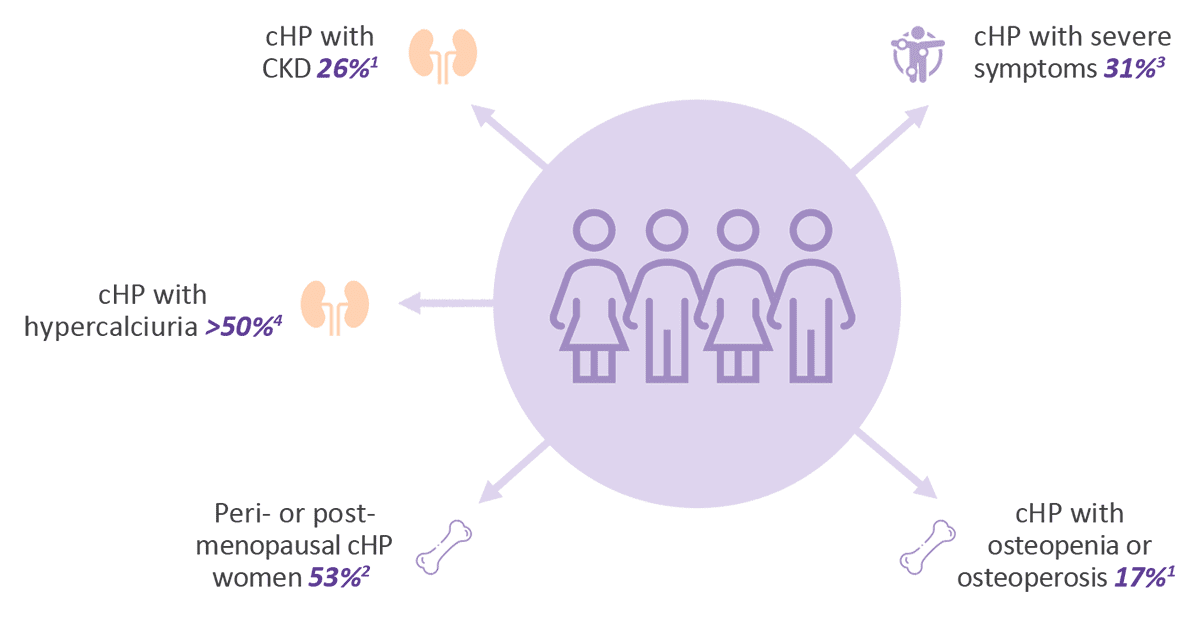
1Proprietary quantitative Market Research, 2021
2Proprietary retrospective Natural History Study, 2020
3Hadker and al., Endocrine Practice, Volume 20 No. 7 July 2014
4Based on clinical baseline patient data (Amolyt Pharma, Ascendis)